Feb 28, 2022
Introduction
The new vaccine that prevents bronchiolitis and was approved this week by the FDA in the United States.It was tested in Argentina, has high efficacy and has already been approved in the United States. It prevents the disease in babies, but pregnant women receive it. The new vaccine that prevents bronchiolitis and was approved this week by the FDA in the United States.
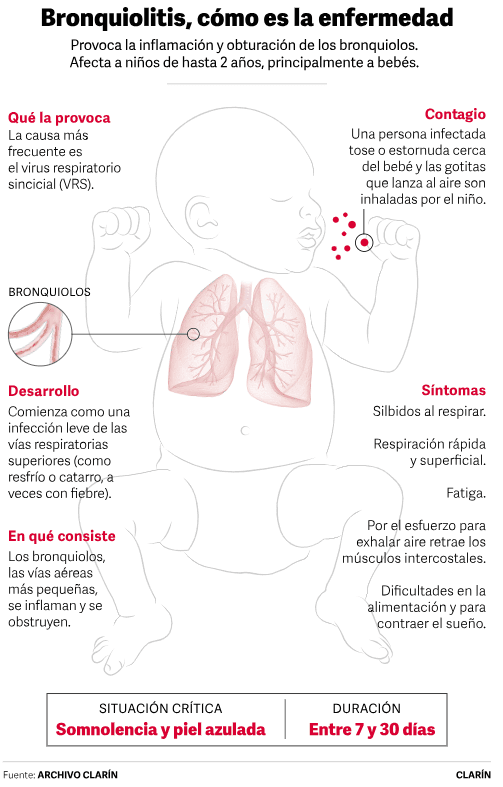
This Monday, the United States became the first country to authorize a vaccine that can spark a revolution in pediatrics : that of the respiratory syncytial virus (RSV) . It is what causes bronchiolitis , a respiratory disease that annually kills 100,000 children under one year of age in the world and that overwhelms pediatric wards every fall/winter.
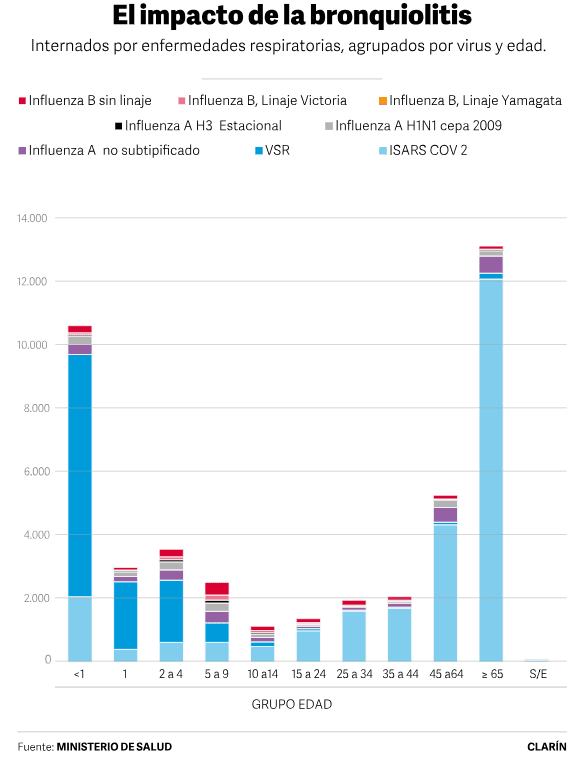
That was what happened in May in Argentina , when greater demand added to the crisis due to the shortage of pediatricians.collapse in care. Because bronchiolitis is one of the main causes of hospitalization in infants : according to data from the latest Epidemiological Bulletin of the Ministry of Health, this season there were more than 143,000 children under two years of age with bronchiolitis . About 11,000 babies under one year old ended up hospitalized for respiratory infections and approximately 70% had respiratory syncytial virus and a collapse in attention was generated . Because bronchiolitis is one of the main causes of hospitalization in infants : according to data from the latest Epidemiological Bulletin of the Ministry of Health, this season there were more than 143,000 children under two years of age with bronchiolitis . About 11,000 babies under one year old ended up hospitalized for respiratory infections and approximately 70% had respiratory syncytial virus .
This new vaccine, from the American laboratory Pfizer, had already been authorized by the US FDA in May for people over 60 years of age . And it received a recommendation from the European health authority, the EMA, but the European Commission has not yet decided whether to approve its marketing on the continent.
As Clarín learned learned From official sources, Pfizer submitted the authorization request to the ANMAT, which is evaluating it and there is still no estimated resolution date.
What there is are results from the clinical trial , in which 7,000 pregnant women participated and in which Argentina was one of the countries that contributed the most volunteers. And they were very positive about its effectiveness : their children's risk of serious illness was reduced by 81.8% in the 90 days after birth and by 69.4% within 180 days after birth. In a subgroup in which they received the vaccine between 32 and 36 weeks of gestation, the risk was reduced even further: 91.1% at 90 days and 75.5% at 180 days . That is why the FDA's indication is to apply the vaccine between those weeks of gestation.
Among the most common side effects in vaccinated pregnant women are discomfort at the injection site, headache or muscle pain, and nausea. Preeclampsia, increased blood pressure in pregnancy, was reported in 1.8% of cases compared to 1.4% of those receiving a placebo. There were also more premature births (5.7% vs. 4.7%), so the FDA called for further study of these two issues.
How the vaccine works
Gonzalo Pérez Marc is the principal investigator of the study that he led from the Central Military Hospital, but in which centers throughout the country also participated. He is ecstatic: he says that bronchiolitis “is not just any disease” and that all doctors when they begin their Pediatric residency in the middle of the year, spend three months without sleep battling RSV . For him, checking this virus also became an obsession in his role as a scientist: more than half of the 15 years that he has been doing research were dedicated to RSV.
He explains that the RSV vaccine is applied to pregnant women because babies “are born with their immune system regulated in such a way that they do not respond with antibodies to everything new in the world because they would develop hyper allergies, they would not be able to live. These diseases are especially harmful in immature lungs . That's why you want them to be protected from birth."

The same thing happens with the influenza vaccine: the aim is for the mother to generate a good amount of antibodies, pass them through the placenta, and the baby is born with mature antibodies. “The challenge is threefold. Achieve a good immune response in the mother, that the vaccine is safe, and that there is a good transplacental passage. From there you set certain milestones and evaluate after three months and six months,” she explains.
What will happen after six months? As we already learned with Covid, with the vaccine rolling out effectiveness, which is the effectiveness measured in the real world. “As in all respiratory viruses, after six months the antibodies decline. But what you are primarily looking to cover is up to six months, with the highest risk of serious illness ,” he points out. Returning to the Epidemiological Bulletin, the data supports this: in the age group of 12 to 24 months, there were about 2,000 hospitalized with RSV, and a similar number between two and four years.Its effectiveness will be evaluated, which is the effectiveness measured in the real world. “As in all respiratory viruses, after six months the antibodies decline. But what you are primarily looking to cover is up to six months, with the highest risk of serious illness ,” he points out. Returning to the Epidemiological Bulletin, the data supports this: in the age group of 12 to 24 months, there were about 2,000 hospitalized with RSV, and a similar number between two and four years.
Roberto Debbag, president of the Latin American Society of Pediatric Infectious Diseases, agrees on the impact of the respiratory syncytial virus and the importance of having a vaccine. “One in three children or more in Latin America will suffer from bronchiolitis in their first year of life,” he says categorically. And he points out that RSV along with pneumococcus and the Haemophilus influenzae bacteria are the three respiratory diseases that cause the highest mortality at that stage of life.
If serious illness is reduced, says Debbag, there are fewer hospitalized patients and greater capacity of health systems to care for other pathologies. But it adds another important variable, which Pérez Marc also warns about: the number of children who die from RSV without knowing it .
“There is a percentage of children who do not reach the health system and who die, and who belong to the vulnerable populations of Latin America and Argentina: poor, ethnic and migrant children,” he warns. For him, the new vaccine “is a tool that will change the history of morbidity and mortality from RSV.” And it adds, as Pérez Marc also highlighted, that the application of a single-dose monoclonal antibody is advancing in the world's regulatory agencies, which would allow a preventive strategy to be drawn up to confront this virus.
Pérez Marc explains that in the case of children who have risk conditions, they will be able to complement the vaccination with this new drug once the vaccine antibodies naturally decline. And he anticipates that the research does not end here: his same group will begin testing the same vaccine in the pediatric population next year , to have a complete preventive “combo.”
In this uncertain electoral context, no one can predict what will happen with access to this vaccine , if it finally meets all the requirements for the ANMAT to approve it. There are no details on how much it would cost, and the laboratory did not provide information about it. The incorporation of a vaccine into the national calendar is analyzed by the National Immunization Commission , which is the one that recommends it or not so that the Ministry of Health then determines it based on health strategies and their cost-effectiveness.
The specialist explains that this vaccine is a pre-fusion protein (one of those used by the virus before joining the cell), which makes it much more stable than those developed previously, and that it can be easily stored in the refrigerator. In addition, another vaccine is being studied, using messenger RNA technology.
Link to the original note: https://www.clarin.com/sociedad/bronquiolitis-vacuna-analiza-anmat-poder-revolucion-pediatria_0_WGQSHXEijY.html
